ARSTECHNICA.COM
Scientists made a stretchable lithium battery you can bend, cut, or stab
Bend it like a battery
Scientists made a stretchable lithium battery you can bend, cut, or stab
Its performance isn't great, but its endurance is similar to standard lithium-ion.
Jacek Krywko
–
Apr 14, 2025 3:30 pm
|
13
Story text
Size
Small
Standard
Large
Width
*
Standard
Wide
Links
Standard
Orange
* Subscribers only
Learn more
The Li-ion batteries that power everything from smartphones to electric cars are usually packed in rigid, sealed enclosures that prevent stresses from damaging their components and keep air from coming into contact with their flammable and toxic electrolytes. It’s hard to use batteries like this in soft robots or wearables, so a team of scientists at the University California, Berkeley built a flexible, non-toxic, jelly-like battery that could survive bending, twisting, and even cutting with a razor.
While flexible batteries using hydrogel electrolytes have been achieved before, they came with significant drawbacks. “All such batteries could [only] operate [for] a short time, sometimes a few hours, sometimes a few days,” says Liwei Lin, a mechanical engineering professor at UC Berkeley and senior author of the study. The battery built by his team endured 500 complete charge cycles—about as many as the batteries in most smartphones are designed for.
Power in water
“Current-day batteries require a rigid package because the electrolyte they use is explosive, and one of the things we wanted to make was a battery that would be safe to operate without this rigid package,” Lin told Ars. Unfortunately, flexible packaging made of polymers or other stretchable materials can be easily penetrated by air or water, which will react with standard electrolytes, generating lots of heat, potentially resulting in fires and explosions. This is why, in 2017, scientists started to experiment with quasi-solid-state hydrogel electrolytes.
These hydrogels were made of a polymer net that gave them their shape, crosslinkers like borax or hydrogen bonds that held this net together, a liquid phase made of water, and salt or other electrolyte additives providing ions that moved through the watery gel as the battery charged or discharged.
But hydrogels like that had their own fair share of issues. The first was a fairly narrow electrochemical stability window—a safe zone of voltage the battery can be exposed to. “This really limits how much voltage your battery can output,” says Peisheng He, a researcher at UC Berkeley Sensor and Actuator Center and lead author of the study. “Nowadays, batteries usually operate at 3.3 volts, so their stability window must be higher than that, probably four volts, something like that.” Water, which was the basis of these hydrogel electrolytes, typically broke down into hydrogen and oxygen when exposed to around 1.2 volts. That problem was solved by using highly concentrated salt water loaded with highly fluorinated lithium salts, which made it less likely to break down. But this led the researchers straight into safety issues, as fluorinated lithium salts are highly toxic to humans.
The challenge Lin, He, and their colleagues set for themselves was making a hydrogel battery with a wide electrochemical window—ideally above 3 volts—that would not cause severe chemical burns when damaged.
Water-scarce hydrogels
The chemistry of the battery they made started with a polymer that has both positive and negative charges (these are termed “zwitterionic”) as the structural net. Water molecules form hydrogen bonds with any charged parts, while lithium ions are attracted to its negatively charged parts. This way, the zwitterionic polymer could bind water tightly enough to prevent it from splitting at higher voltages while still releasing lithium ions when needed. The team then used acrylic acid as the gel’s crosslinker and an electrolyte with a fluorine-free lithium salt to provide lithium ions.
Credit:
He et al.
The salt also played an additional role: It pulled water from the air. The usual way of getting the “hydro” part into a hydrogel is soaking the crosslinked, hydrophilic polymer in water; it will typically reach 80 percent water content. Lin, He, and their colleagues didn’t want their hydrogel to contain that much water because of the water-splitting issue. So they just left it to absorb water from ambient moisture.
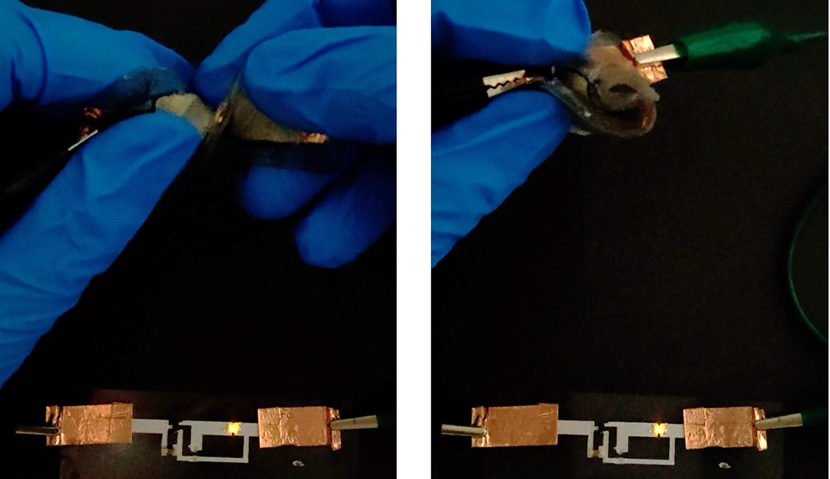
The result was a water-scarce hydrogel electrolyte that contained only 19 percent water and remained stable in a normal room humidity of 50 percent or so. Once the electrolyte was ready, the team added electrodes and built a fully functional battery powering a printed circuit with a few LED lights. The battery could work for over a month without sealed packaging and operate at over 3.1 volts without much water splitting—a voltage close to that used by commercial batteries.
Despite this success, they started inflicting all sorts of punishment on it.
Self-healing soft batteries
The soft, jelly-like battery could power the LEDs when it was twisted 180 degrees, bent, punctured with a needle, and cut with a razor. It could even self-heal and get back to 90 percent of its original capacity after the team cut it in half, although this required putting it back together and warming it in an oven. Because the electrolyte reached its equilibrium state in ambient air, it didn’t degrade due to air exposure as other hydrogel electrolytes did and remained functional after 500 complete charge cycles.
There were some drawbacks, though. A commercial battery designed for 500 cycles, in principle, should retain 80 percent of its capacity once those 500 cycles are done. Lin and He’s soft battery retained around 60 percent, which leaves lots of room for improvement. Another problem was the energy density. “When you compare this with state-of-the-art batteries we have today, we achieved roughly one-tenth of their capacity,” He says. “So, I think definitely we can try to optimize more towards energy density. Depending on the application, we may sacrifice some properties, like self-healing.”
Lin, however, thinks that the relatively low energy density does not tell the full story. “Your smartwatch is powered by a battery, but a band for this watch today performs only the mechanical function,” Lin says. “If you can replace the band with our battery, you have more area, more volume to work with. Instead of needing a recharge once a day, it could perhaps work for, like, a week.”
Science Advances, 2025. DOI: www.science.org/doi/10.1126/sciadv.adu3711
Jacek Krywko
Associate Writer
Jacek Krywko
Associate Writer
Jacek Krywko is a freelance science and technology writer who covers space exploration, artificial intelligence research, computer science, and all sorts of engineering wizardry.
13 Comments