ARSTECHNICA.COM
New RSV vaccine, treatment linked to dramatic fall in baby hospitalizations
Good news
New RSV vaccine, treatment linked to dramatic fall in baby hospitalizations
CDC study finds big declines in hospitalizations—and they may be underestimates.
Beth Mole
–
May 8, 2025 5:54 pm
|
11
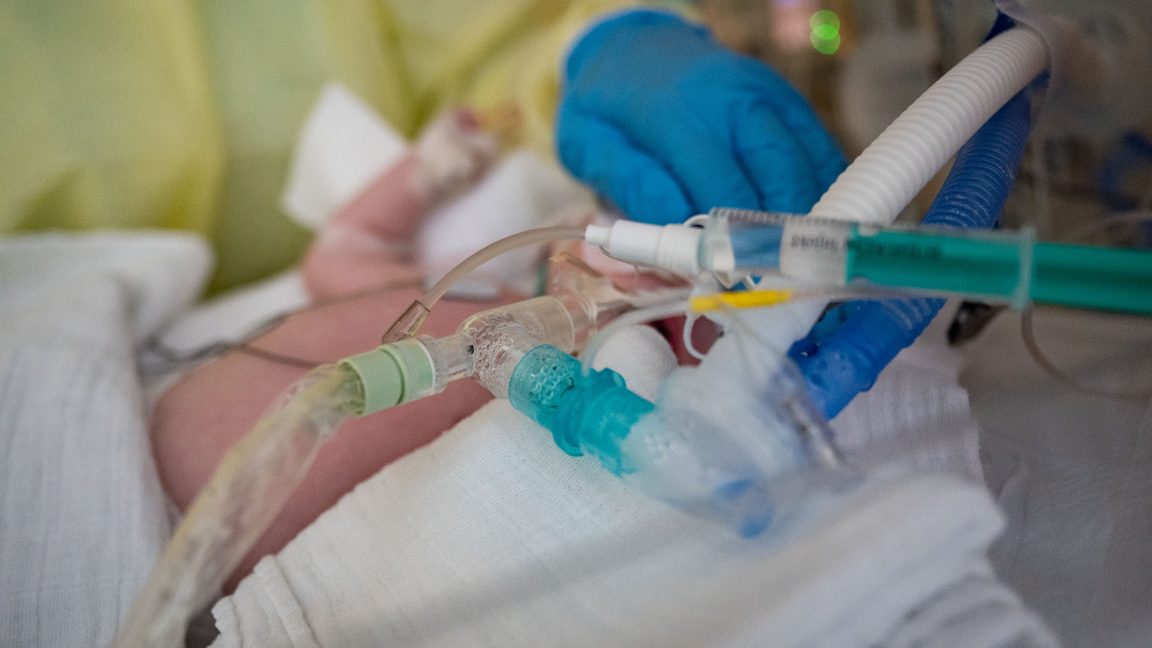
An intensive care nurse cares for a patient suffering from respiratory syncytial virus (RSV), who is being ventilated in the children's intensive care unit of the Olga Hospital of the Stuttgart Clinic in Germany.
Credit:
Getty | picture alliance
An intensive care nurse cares for a patient suffering from respiratory syncytial virus (RSV), who is being ventilated in the children's intensive care unit of the Olga Hospital of the Stuttgart Clinic in Germany.
Credit:
Getty | picture alliance
Story text
Size
Small
Standard
Large
Width
*
Standard
Wide
Links
Standard
Orange
* Subscribers only
Learn more
Far fewer babies went to the hospital struggling to breathe from RSV, a severe respiratory infection, after the debut of a new vaccine and treatment this season, according to an analysis published today by the Centers for Disease Control and Prevention.
RSV, or respiratory syncytial (sin-SISH-uhl) virus, is the leading cause of hospitalization for infants in the US. An estimated 58,000–80,000 children younger than 5 years old are hospitalized each year. Newborns—babies between 0 and 2 months—are the most at risk of being hospitalized with RSV. The virus circulates seasonally, typically rising in the fall and peaking in the winter, like many other respiratory infections.
But the 2024–2025 season was different—there were two new ways to protect against the infection. One is a maternal vaccine, Pfizer's Abrysvo, which is given to pregnant people when their third trimester aligns with RSV season (generally September through January). Maternal antibodies generated from the vaccination pass to the fetus in the uterus and can protect a newborn in the first few months of life. The other new protection against RSV is a long-acting monoclonal antibody treatment, nirsevimab, which is given to babies under 8 months old as they enter or are born into their first RSV season and may not be protected by maternal antibodies.
For the new study, CDC researchers looked at RSV hospitalization rates across two different RSV surveillance networks of hospitals and medical centers (called RSV-NET and NVSN). They compared the networks' hospitalization rates in the 2024–2025 RSV season to their respective rates in pre-pandemic seasons between 2018 and 2020. The analysis found that among newborns (0–2 months), RSV hospitalizations fell 52 percent in RSV-NET and 45 percent in NVSN compared with the rates from the 2018–2020 period. However, when the researcher excluded data from NVSN's surveillance site in Houston—where the 2024–2035 RSV season started before the vaccine and treatment were rolled out—there was a 71 percent decline in hospitalizations in NVSN.
For a broader group of infants—0 to 7 months old—RSV-NET showed a 43 percent drop in hospitalizations in the 2024–2025 RSV season, and NVSN saw a 28 percent drop. Again, when Houston was excluded from the NVSN data, there was a 56 percent drop.
Lastly, the researchers looked at hospitalization rates for toddlers and children up to 5 years old, who wouldn't have been protected by the new products. There, they saw RSV hospitalization rates were actually higher in the 2024–2025 season than in the pre-pandemic years. That suggests that the latest RSV season was more severe, and the drops in infant hospitalizations may be underestimates.
Beth Mole
Senior Health Reporter
Beth Mole
Senior Health Reporter
Beth is Ars Technica’s Senior Health Reporter. Beth has a Ph.D. in microbiology from the University of North Carolina at Chapel Hill and attended the Science Communication program at the University of California, Santa Cruz. She specializes in covering infectious diseases, public health, and microbes.
11 Comments