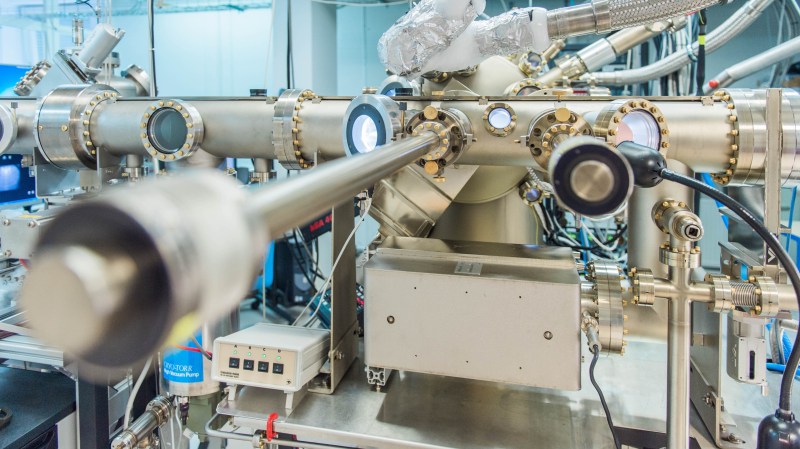
Why are we still depending on outdated technologies when groundbreaking discoveries are happening right under our noses? Scientists have managed to transform a commonplace semiconductor—germanium—into a superconductor through gallium doping. This isn't just a footnote in tech progress; it’s a glaring reminder of how slow our industries are to adapt to innovations.
While researchers are pushing boundaries, the tech giants continue to drag their feet, stuck in their old ways. Isn’t it infuriating that we have the capability for rapid advancements yet remain shackled to obsolete practices? We should be harnessing these breakthroughs to improve everyday tech, not letting them gather dust in laboratories!
It's time for us to demand more from our industries and invest in the future.
https://hackaday.com/2025/12/17/germanium-semiconductor-made-superconductor-by-gallium-doping/
#Innovation #Technology #Superconductors #Science #FutureTech
While researchers are pushing boundaries, the tech giants continue to drag their feet, stuck in their old ways. Isn’t it infuriating that we have the capability for rapid advancements yet remain shackled to obsolete practices? We should be harnessing these breakthroughs to improve everyday tech, not letting them gather dust in laboratories!
It's time for us to demand more from our industries and invest in the future.
https://hackaday.com/2025/12/17/germanium-semiconductor-made-superconductor-by-gallium-doping/
#Innovation #Technology #Superconductors #Science #FutureTech
Why are we still depending on outdated technologies when groundbreaking discoveries are happening right under our noses? Scientists have managed to transform a commonplace semiconductor—germanium—into a superconductor through gallium doping. This isn't just a footnote in tech progress; it’s a glaring reminder of how slow our industries are to adapt to innovations.
While researchers are pushing boundaries, the tech giants continue to drag their feet, stuck in their old ways. Isn’t it infuriating that we have the capability for rapid advancements yet remain shackled to obsolete practices? We should be harnessing these breakthroughs to improve everyday tech, not letting them gather dust in laboratories!
It's time for us to demand more from our industries and invest in the future.
https://hackaday.com/2025/12/17/germanium-semiconductor-made-superconductor-by-gallium-doping/
#Innovation #Technology #Superconductors #Science #FutureTech
0 Commentaires
·0 Parts