Researchers reveal a new 3D printing method for scaling fish fillet production
3dprintingindustry.com
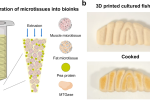
Researchers from Ocean University of China have developed a scalable method for producing lab-grown fish fillets using edible porous microcarriers (EPMs) and bioprinting.With growing concerns over overfishing, climate change, and food security, cultivated seafood has been gaining attention as an alternative to traditional aquaculture. But scaling up production while keeping the texture, structure, and nutritional profile intact remains a challenge. Now, a study published in Nature Communications details how fish muscle and fat cells can be efficiently expanded, structured into a bioink, and 3D printed into fillets that closely mimic wild-caught seafood.a) Properties of microcarrier-based cellular microtissues bioinks. The diagram was created using BioRender. b) The appearance of raw and cooked 3D printed cultured fish fillet prototypes. Image via Ocean University of China.Porous microcarriers enable high-density cell expansionAs per the team, this research focused on optimizing gelatin-based EPMs to improve cell adhesion, growth, and differentiation. By introducing sodium chloride (NaCl) during cryogenic crosslinking, scientists controlled ice crystal formation to fine-tune pore size, creating a scaffold with the right porosity for high-density cell culture.With this method, muscle satellite cells (SCs) and adipose-derived stem cells (ASCs) from large yellow croakers expanded to densities of 6.25 105 and 5.77 105 cells/mL marking a 499-fold and 461-fold increase, respectively.To test scalability, the researchers moved from 125-milliliter spinner flasks to a 4-liter bioreactor, where consecutive expansion cycles kept cell viability above 80%. The collagenase digestion method proved the most effective for transferring cells onto fresh microcarriers, maintaining uniform distribution and preventing cell loss.RNA sequencing confirmed that these expanded cells retained their ability to differentiate, with notable increases in genes related to muscle growth, extracellular matrix remodeling, and cell cycle regulation.Once matured, muscle and fat microtissues were mixed into a bioink, which was extruded through a commercial 3D bioprinter to create structured fish fillets measuring 100 mm in length and 15 mm in height. The printed fillets had layered textures similar to natural fish muscle and developed a browned surface after cooking due to the Maillard reaction.Analysis showed that the printed fish fillets retained moisture (~70%) and had a weight loss of ~35%, similar to conventional fish. However, textural properties like chewiness and cohesiveness were slightly lower, leaving room for refinement in food structuring.Nutritionally, the cultured fish had 8.5 grams more protein per 100 grams than its natural counterpart, with 68.92% less fat and an 87.93% reduction in cholesterol. The omega-3 fatty acid profile remained stable, though sodium content was higher, exceeding that of natural fish by 192.7 mg/100 g. A 51% increase in essential amino acids was also observed, while flavor compound analysis highlighted differences in volatile organic profiles, suggesting areas for further optimization in taste and aroma.Although scaling up remains a challenge, researchers estimate that a 100-liter bioreactor could yield around 750 grams of cultured fish per batch, signaling commercial potential for EPM-based cell expansion.While this study demonstrates significant progress in structured cultivated seafood, fine-tuning fiber alignment, bioink composition, and production costs will be key to making lab-grown fish market-ready.This research highlights the feasibility of scalable cultivated fish production, positioning bioprinting and high-density cell culture as tools that could reshape the future of seafood. As technology advances, lab-grown fish may soon offer a sustainable alternative to meet global demand while reducing pressure on marine ecosystems.3D printing fishes on the riseEfforts to improve alternative seafood are expanding, with companies exploring unique approaches. Last year, Vienna-based food-tech company Revo Foods teamed up with Belgian-based Paleo to make its 3D printed vegan salmon even more realistic.Revo Foods 3D printed salmon filet. Photo via Revo Foods. Backed by a 2.2M grant from the EUs Eureka Eurostars program, the two-year project, which began in August 2024, will see Paleo developing a specially fermented Myoglobin protein to enhance the taste, texture, and nutritional value of Revo Foods salmon alternative. Typically found in animal muscle, Myoglobin will be recreated without animal use to add color, iron, and aroma. Adding to this development, Revo Foods claimed its 3D printing process cuts water use by 90% and CO2 emissions by 75%.Back in 2020, Legendary Vish, a startup founded by a group of international students, was working to bring 3D printed plant-based fish alternatives to market. The idea stemmed from a 2017 EU-funded research project, where the team developed an extrusion-based method to create structured salmon fillets using plant-based bio-inks.Their goal was to offer a sustainable seafood alternative amid growing concerns over overfishing and environmental damage. As they sought investment to scale production, they also explored regulatory approval and potential partnerships to expand into Scandinavian and European markets.What3D printing trendsshould you watch out for in 2025?How is thefuture of 3D printingshaping up?To stay up to date with the latest 3D printing news, dont forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.While youre here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.Featured image shows a) Properties of microcarrier-based cellular microtissues bioinks. The diagram was created using BioRender. b) The appearance of raw and cooked 3D printed cultured fish fillet prototypes. Image via Ocean University of China.
0 Commentarios
·0 Acciones
·49 Views


