3DPRINTINGINDUSTRY.COM
Faster and ethical bone repair testing: Swanseas breakthrough
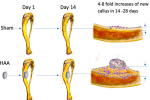
Researchers at Swansea University have developed a novel approach to testing biomaterials for bone regeneration, offering both speed and ethical refinement.Published in Bioactive Materials journal, and led by Dr. Zhidao Xia, a team from Swanseas Medical School and Faculty of Science and Engineering has introduced a murine tibial periosteal ossification model that eliminates the need for traditional methods involving fractures or critical sized defects.In doing so, they significantly reduce animal suffering while enabling biomaterial evaluation in as little as 14 days, with cortical bone remodeling completed by 28 days.Dr. Xia explained, Our invention bridges the gap between synthetic substitutes and donor bone. Weve shown that its possible to create a material that is safe, effective, and scalable to meet global demand. This could end the reliance on donor bone and tackle the ethical and supply issues in bone grafting.Alongside Swansea, several institutions worldwide contributed to this project. These include the likes of Huazhong University of Science and Technology, and Xiangyang Central Hospital from China, Johns Hopkins University School of Medicine and the University of Rochester from USA, McGill University from Canada, Oxford Instruments NanoAnalysis, The Open University, the University of Oxford, and the University of Sheffield from UK.A murine animal model for rapid assessment of osteogenesis. Image via Swansea University.Periosteal model improves bone repair testingCentral to the models success is its focus on the tibial periosteum, where osteogenic cells naturally drive bone repair. Implanting biomaterial scaffolds between the tibia and surrounding muscle tissue has yielded notable results, including trabecular bone growth within two weeks.By the fourth week, this bone remodels into a new cortical layer. These findings, measured through micro computed tomography and histological analysis, revealed an exceptional eightfold increase in tibial thickness compared to control groups.One of the materials tested in this model is a hydroxyapatite aragonite (HAA) scaffold, a blend of hydroxyapatite widely used in bone grafts and calcium carbonate, which enhances biodegradation and supports bone growth.The scaffold gradually breaks down over six to twelve months, creating space for new bone tissue to grow. Researchers also experimented with zoledronate, a clinical bisphosphonate, finding that its addition significantly increased cortical bone formation, demonstrating its potential in enhancing biomaterial performance.Unlike ectopic methods where bone is generated in unnatural locations, this approach maintains the materials direct contact with both bone and muscle. This proximity replicates the complex environments typically encountered in orthopaedic applications, making the findings highly relevant for clinical scenarios.Validation of the model across species including rats and minipigs confirmed consistent outcomes. The HAA scaffold not only facilitated bone regeneration but also degraded fully within six months, according to the researchers.Ethical refinements and future directionsA notable advantage of the model lies in its streamlined testing process. Eliminating the need for large bone defects or fractures significantly reduces the invasiveness and duration of experiments.While the technique is particularly suited for early stage screening of biomaterials, larger animal trials and traditional fracture models are still required for regulatory approval and testing in complex defect scenarios.Further investigations aim to clarify the role of specific cells such as periosteal stem cells in the observed rapid bone formation. Improvements to micro computed tomography software are also needed to better analyze biomaterial performance, as current tools lack precision for evaluating these interactions.Despite these challenges, the research highlights calcium carbonates pivotal role in accelerating bone growth. Its faster dissolution compared to hydroxyapatite enables bone cells to infiltrate the scaffold more efficiently, although the exact mechanisms warrant additional study.Importantly, this model aligns with ethical research principles by significantly reducing animal suffering. The absence of induced fractures or large bone defects minimizes harm, offering a refined and humane method for early-stage biomaterial screening.While traditional fracture models and large animal trials remain essential for regulatory approval, this approach could streamline preclinical testing and reduce the number of animals required.The size and quality of the newly-formed bone calluses was compared through 3D analysis of the micro CT scanning data. Image via Swansea University.Alternative bone regeneration methodsGlobally, research groups have explored alternative approaches, each offering unique advantages in tackling the challenges of bone repair.Two years ago, researchers from Carnegie Mellon University and the University of Connecticut developed 3D printed calcium phosphate graphene (CaPG) scaffolds for future bone regeneration applications.Tested in vitro and in vivo, the scaffolds demonstrated osteogenic potential by supporting stem cell differentiation and regenerating bone in animal models. Using a Direct Ink Writing method, the CaPG scaffolds were found to biodegrade and resorb in vivo, promoting tissue regeneration without adverse effects on vital organs.Published in Nature, this study highlights CaPGs potential as a cost-effective, customizable, and resorbable alternative to traditional bone grafts.Back in 2017, researchers from Tianjin University, the Chinese Academy of Sciences, and the University of Hong Kong developed a 3D printable hydrogel-nanoclay composite designed to support bone cell growth.Published in ACS Biomaterials Science & Engineering, this material addresses bone defects caused by trauma, deformities, or tumor removal. In tests, the material promoted bone regeneration in tibia defects of live rats, significantly outperforming placebo treatments over eight weeks.Offering structural support and nutrition, the scaffold mimicked the extracellular matrix and had a potential to offer precision and individualized repair of load-bearing bone defects.What 3D printing trends do the industry leaders anticipate this year?What does the Future of 3D printing hold for the next 10 years?To stay up to date with the latest 3D printing news, dont forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.While youre here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.Featured image shows a murine animal model for rapid assessment of osteogenesis. Image via Swansea University.
0 Yorumlar
0 hisse senetleri
154 Views


